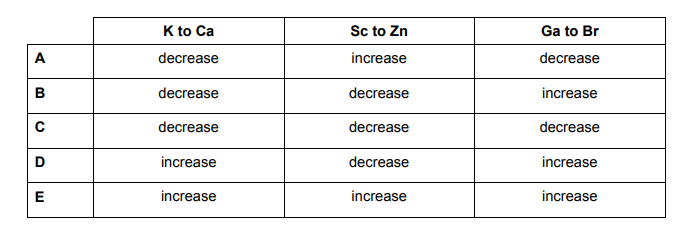
Which of the following shows how the atomic radius of the elements changes on crossing from left to right in the row of the Periodic Table from potassium to bromine?
1 Like
To know how to answer this question and similar questions on the IMAT, you will have to memorize the periodic trends;
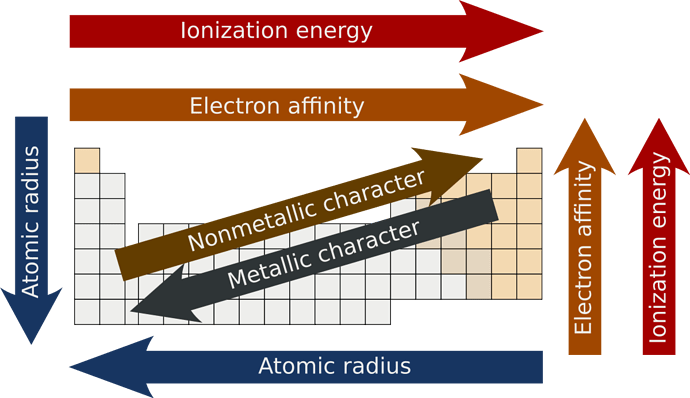
The atomic radius as you can see increases from the right upper corner all the way to the bottom left of the table.
A higher effective nuclear charge causes greater attraction to the electrons, pulling the electron cloud closer to the nucleus which results in a smaller atomic radius.
We will cover the rest of the periodic trends in future questions.
4 Likes