Which one of the following compounds can be made from ethanol using only a substitution reaction?
A. Ethene
B. Ethanal
C. Ethanoic acid
D. Ethoxyethane
E. Bromoethane
Which one of the following compounds can be made from ethanol using only a substitution reaction?
A. Ethene
B. Ethanal
C. Ethanoic acid
D. Ethoxyethane
E. Bromoethane
To compare we can quickly say:
Ethene can be made by an elimination reaction. Therefore A is incorrect.
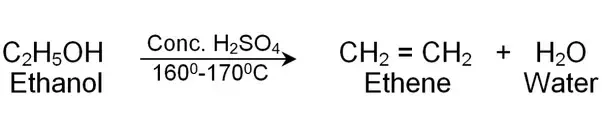
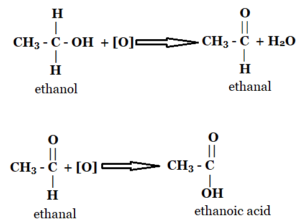
Ethanal and Ethanoic Acid can be formed by the oxidation of Ethanol. Therefore B and C are incorrect.
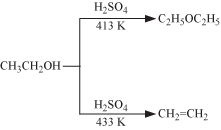
Ethoxyethane can be formed by the dehydration of Ethannol. Therefore D is also incorrect.
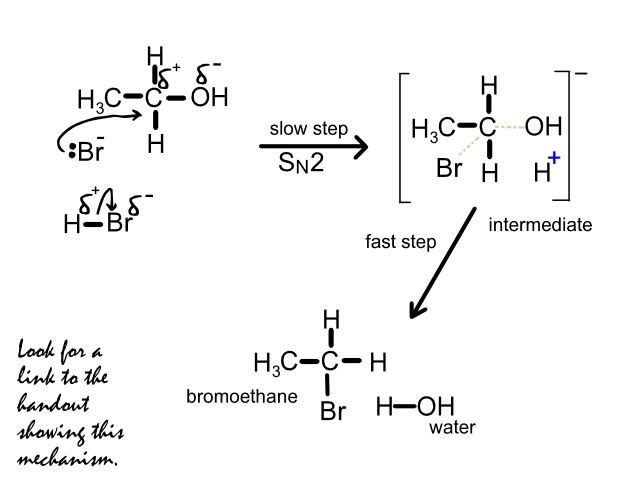
Bromoethane can only be formed by a simple substitution reaction of Ethanol, which makes E our answer.
It’s a tough question, isn’t it?![]()
![]()
Can someone please explains why the reaction between ethanol and O is an oxidation reaction? Because the atoms in ethanol have covalent bonds and the atoms are neutral and not charged.
Oh! Thank you very much🙏