The positions of some elements in the Periodic Table are shown below.
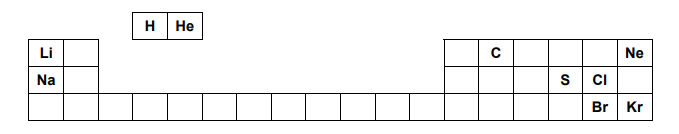
Which two of the elements shown react most energetically with each other?
A. Li and Kr
B. Ne and Na
C. C and He
D. Li and Br
E. Na and Cl
The positions of some elements in the Periodic Table are shown below.

Which two of the elements shown react most energetically with each other?
A. Li and Kr
B. Ne and Na
C. C and He
D. Li and Br
E. Na and Cl
The answer to this particular question is easy to figure out if we keep in mind that the group of alkali and halogens react aggressively. The reason that the answer is E and not D is that the trend of reactivity regarding the alkali goes from top to bottom and since Sodium is below Lithium, E is the correct answer.
Metal reactivity decreases from left to right across periods and increases down groups . nonmetallic characteristics increase from left to right and decrease down groups.
Nonmetal reactivity increases from left to right and decreases down groups.