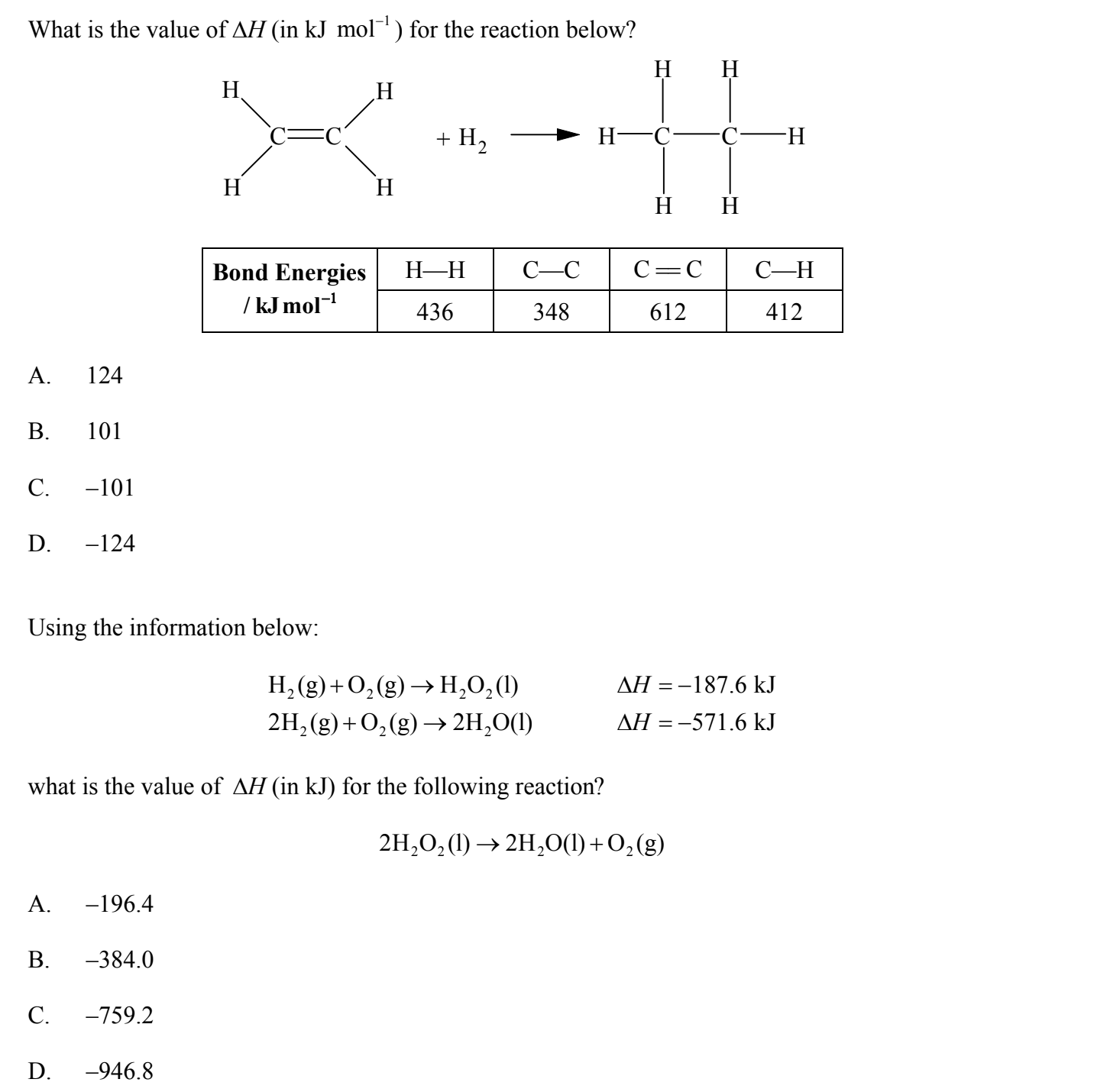
1)so for the first question i just found the sum of bond energies on both sides and then found the difference between them .
612+(412x4) + 436 ----> (348) + (412x6)
reactant=2696 product=2820
reactant - product = -124 kJ mol^-1
- we have to drive the enthalpy of the new reaction from the above enthalpy given . shortly we can just reverse the first rxns enthalpy by changing the sign to +187.6kJ because H2O2 is on the reactant side for the new rxn given, then multiply it by 2 (+187.6x2) because it has a coefficient of 2.
we dont need to change anything for the second rxn as it already has H20 on the product side and with a coefficient of 2 so we leave it as (-571.6kJ).
at the end we just add them up to get the new value .
(+187.6x2)+(-571.6)=-196.4
for the second question the answer is D -946.8
but i got what you meant. i think you made a mistake with h2o and h2o2
thanks!!
Hey thanks for letting me know however I seriously cant find D for the second question , are you sure?
ah sorry i think i made a mistake with the answer key. yes you are right!
1 Like