Hello!
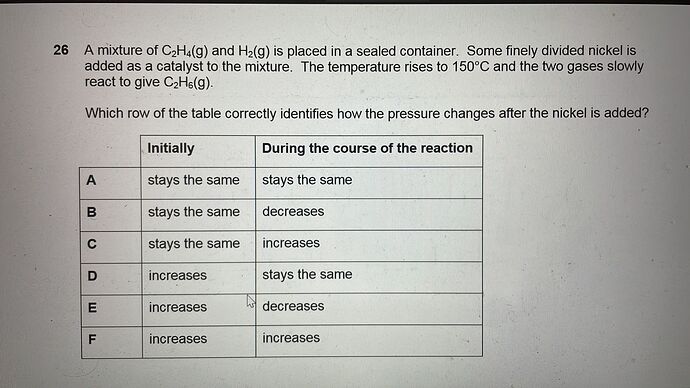
26 A mixture of C2H4(g) and H2(g) is placed in a sealed container. Some finely divided nickel is added as a catalyst to the mixture. The temperature rises to 150°C and the two gases slowly react to give C2H6(g).
Which row of the table correctly identifies how the pressure changes after the nickel is added?
Initially
During the course of the reaction
A
stays the same
stays the same
B
stays the same
decreases
C
stays the same
increases
D
increases
stays the same
E
increases
decreases
F
increases
increases
Why does the pressure increases initially?
Thank you ![]()