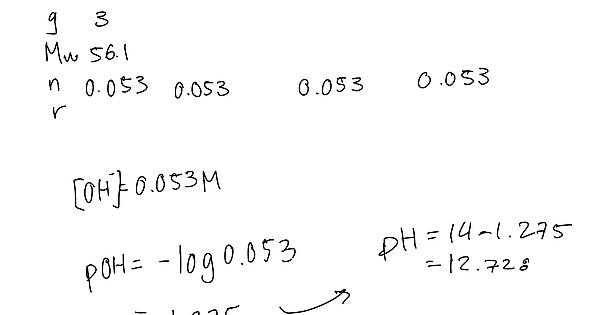did another question and got the right answer using -log[pOH] however when I tried it in the format of 10^-[pOH] I got a different answer. Does anyone know why? My work is attached below and the answer is 12.728.
hey
the formula is (H+) =10^-PH
you have put the concentration of OH- instead of PH
guess you mixed them up
1 Like
Also to find the H+ concentration use Kw = [H+][OH-] = 1.0 X 10-14 when substituting for OH- concentration
1 Like