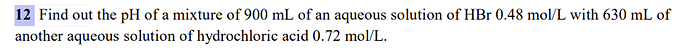hi
for HBr n = C * V = 0,48 * 0,9L = 0,43
for HCl n = 0,63 * 0,72 = 0,45
total n = 0,43 + 0,45 = 0,88
total V = 0,9 + 0,63 = 1,53L
Cf = total n / total V = 0,88/1,53 = 0,6
pH = -log10[10^0,6]
we don’t have a calculator on the exam, but we know the value of the pH will be between 0 and 1 because :
10⁻¹ < 10^0,6 < 10⁰
3 Likes