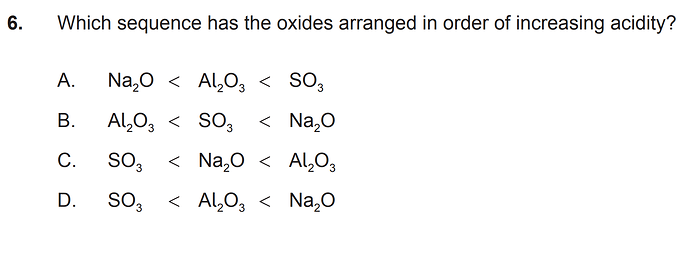hi
strength of oxyacids increase left to right and bottom to top
so Na<Al<S
2 Likes
one question,
if it increases left to right, what about F2O? wouldn’t it be a weak acid because F is strong? what am i missing here ![]()
but oxyacids can’t have metals?
Edit: the choices have Na and Al
that’s a great point, Al is amphoteric so it can still behave as an oxyacid
Na2O shouldn’t be an oxyacid, instead it acts as a base, so it will definitely have the least acidic/oxyacidic character out of the three options given
1 Like