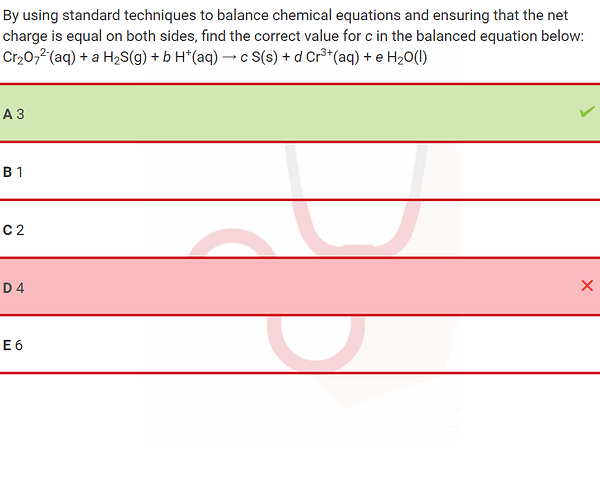
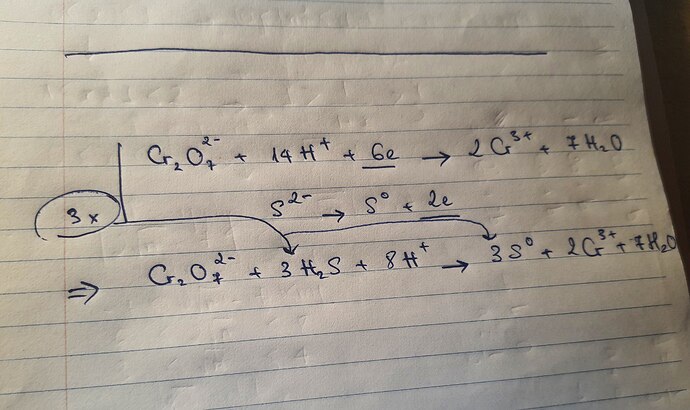hey all, can someone explain why the answer is 3?
Hi, you need to balance the charges as well - 1 molecule of dichromate being key to balancing this - because that means we have 7 H20 and 2 Cr3+ , 6+ on the right side means we will have 8H+ on the left, 14-8, 6H left on the left side of the equation to be balanced - which comes out to 3H2S
You’ll need to balance using 2 half-redox reaction with Cr2O7 2- being the oxidizing agent and S2- (in H2S) reducing agent.
hi ,i did something different and found option a
so
we can see in the products that oxidation number of cr is 3
so i found the oxidation number of cr in cr2o7
(Cr x 2)+(-2)x7=-2 =>2Cr - 14 = -2 => 2Cr=14-2=>2cr=12=>cr=6 so oxidation number of cr in cr207-2 is 6
we can see that from 6 it went to 3 so difference is 6-3=3
we multiply the H2S and S with 3 and a is the correct option