Can someone explain the reasoning behind this answer please!
@callearaya Hey! In my point of view it is because of the fatty acid tails of the phospholipids in the olant membrane.
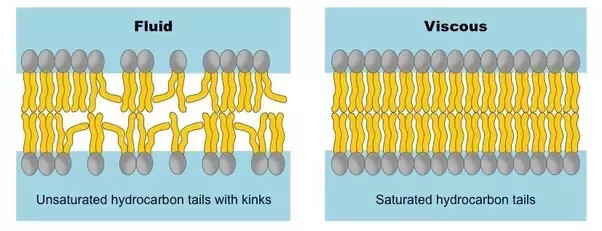
The more double bonds will dicrease the close packing …
The carbon chain in the phospholipids on the membrane helps maintain a particular strength of the cell membrane. The force with which the membrane holds itself together calls the fluidity.
Imagine a water balloon; the balloon’s rubber is the cell’s membrane. The thicker the rubber, the stronger it will be, and the more reliable it will be when we fill water inside, but on the other hand, it will also be harder to fill the water because the rubber is more extensive and less fluid, so it will be difficult for us to transfer water inside (or in the case of the cell, metabolites that we need to move inside the cell)
Because of that, the cell must maintain a certain balance of fluidity in its cell membrane. The temperature changes the fluidity. Colder temperature makes it less fluid because the phospholipids’ hydrocarbon chains are getting closer. To fix that, unsaturated carbon chains will allow “kinks” to form, increasing the distance between the phospholipids. C=C will cause kinks in the chains and increase the distance between each other.
Thank you so much, this analogy helped me a lot!
