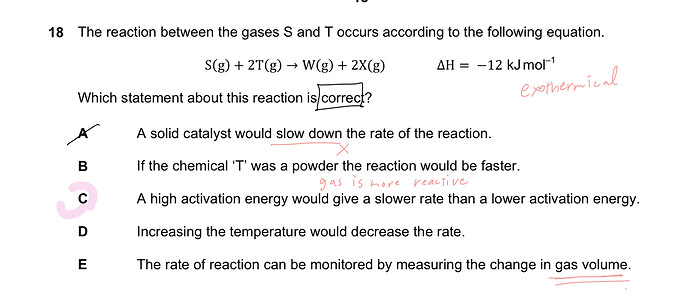
I am struggling with understand of D and E. I already saw Ari video.
This reaction is exothermic. Do The amount of W and X
decrease when increasing the temperature?
Hi!
the reaction isn’t at equilibrium yet
so increasing the temperature favors the reaction, it would increase the rate of formation of W and X
i don’t think we can see a change from monitoring gas volume as both the left and right side have gas components and they have the same coefficients?
2 Likes
Hey guys,
since it’s an open system, what happens if we add more S?
a shift to the right? or maybe we need to add both S and T to shift to the right?
1 Like
We need both S and T. If we add just S, there will be more products UNTIL T becomes limiting then excess S will be present and it wouldn’t become the products. More of both reagents would give more products :))
2 Likes