1 Like
Hi!
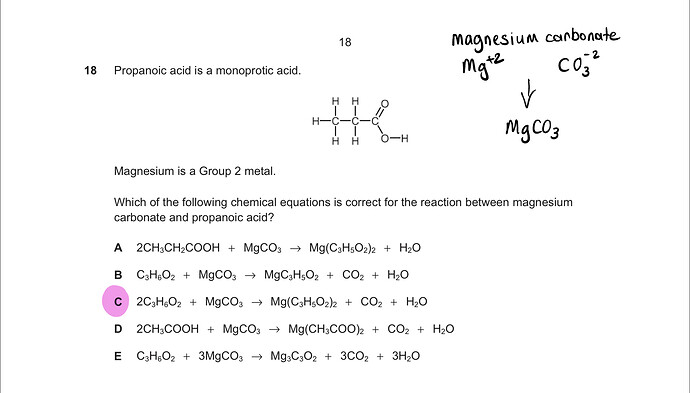
As propanoic acid is a monoprotic acid it will dissociate to form it’s anion and H+ as we know that when we have a metal carbonate + acid we form a metal salt + CO2 + H2O.
Now we can analyze each answer options to eliminate the wrong ones.
A). we can eliminate A for forming the wrong products.
As Mg will form a +2 cation and the anion of propanoic acid has -1 charge we need two propanoic acids for every Mg this eliminates B and even D and E because the correct anion has the formula CH3 CH2 COO- whereas in D this hasn’t got enough H atoms and in E there are no H atoms in the anions.
we are left with C and it’s balanced showing a 2:1 ration b/w acid and and M2+ ion.
answer is thus C
hope it helps:)
5 Likes
thanks so much for the explanation!
2 Likes