Hi!
i find this question a little ambiguous
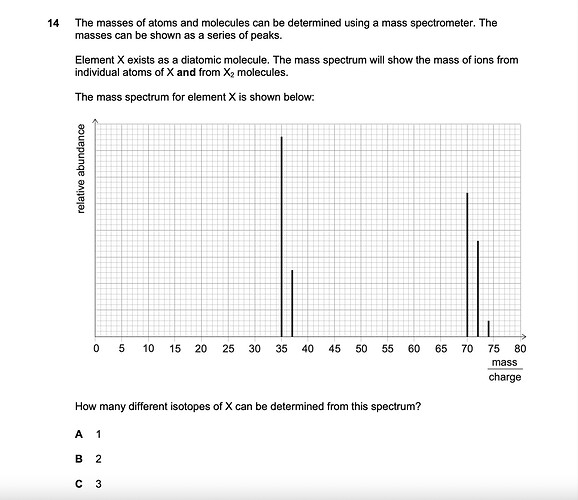
we can deduce that X is chlorine and exists as ³⁵Cl and ³⁷Cl from the first two spikes
the rest of the spikes mean the diatomic molecules are ³⁵Cl³⁵Cl and ³⁵Cl³⁷Cl and ³⁷Cl³⁷Cl
there are two different types of isotopes present
by “how many different” i don’t know if they want us to find the number of atoms or moles in the sample and i’m not sure how to go about that
I believe since X is a diatomic molecule we need to disregard the combinations to find what are the two isotopes of Cl, which would be either 35 or 37 (different neutrons), which is deduced from the two spikes towards the left. Isotope means atom of X unless specified it’s a compound. Is the answer B (2)?