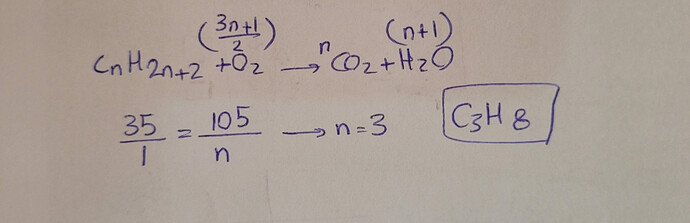22 Complete combustion of 35 cm3
of a straight-chain alkane vapour gave 105 cm3
of carbon
dioxide gas. Both gas volumes were measured at the same temperature and pressure.
Which of the following is the molecular formula of the alkane?
A C2H4
B C2H6
C C3H6
D C3H8
E C4H10