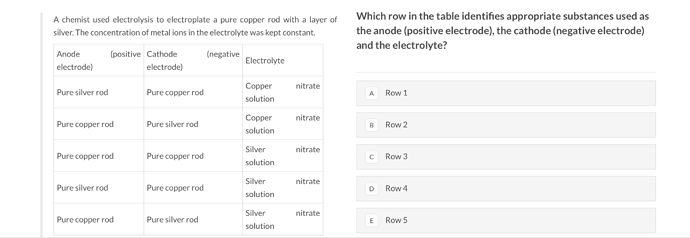
can someone explain this question? how do we determine Anode and Cathode for this question? The answer is D btw.
Hi!
I tried answering to the best i could:
-
reduction potential increases as we go up and to the right on the periodic table
-
Cu is right above Ag in the periodic table, so Cu will be the oxidant that will undergo a reduction.
-
the anode always does oxidation and the cathode always does reduction.
We now know Copper has to be on the cathode side. We can eliminate row 2) and 5) as potential answers.
Let’s look at our half reactions:
- Cu²⁺ + 2e⁻ — Cu (reduction)
- Ag — Ag²⁺ + 2e⁻ (oxidation)
As you can see we will start forming more solid Cu on the rod, while the silver rod will disintegrate. So we will have dissolved Silver in our solution.
We can eliminate row 1).
Row 2) to me doesn’t make any sense, i don’t think we can have have a working electrolytic cell if we have both rods that are the same?
We are left with row 4) as the answer
Hope this helps!