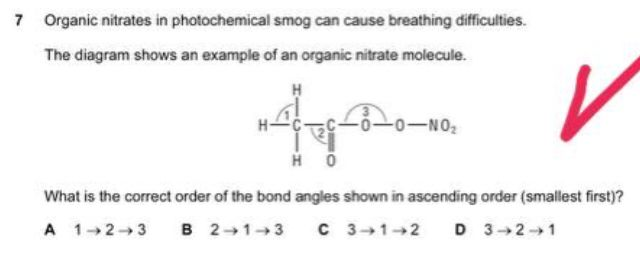
1: 109 degree
2: 120 degree
3: 180 degree
I chose a. But the correct answer is c. How?!

1: 109 degree
2: 120 degree
3: 180 degree
I chose a. But the correct answer is c. How?!
Answer credited to @Asafmen
You’ve forgotten to take the lone pairs into account.
The structure of Bond 3 is not linear, it is a tetrahedron.
Oxygen has 2 lone pairs, so it has angles slightly smaller than the tetrahedron of 1 (as in less than 109.5).
Thus, the answer will be C.
Use this to review the VSEPR and Hybridization, for future reference.