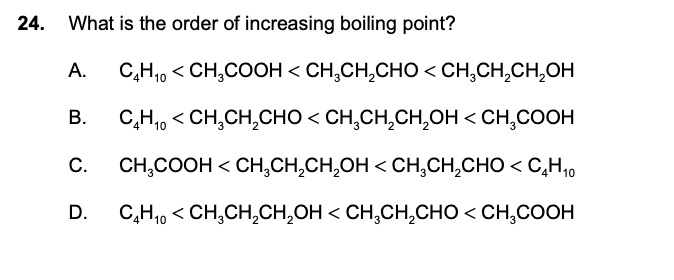
answer: B
Among organic compounds carboxylic acid have the highest boiling point because of strong intramolecular hydrogen bonding, alcohols come second as they also possess intramolecular h- bonding but the O-H bond in carboxylic acids is more strongly polarised due to the presence of adjacent electron-withdrawing carbonyl groups and therefore forms diemers.
Alcohols and acids show intermolecular hydrogen bonding. But no intermolecular H-bonding occur in aldehydes. Therefore attractive forces in aldehydes are less thus have low boiling points.
Hydrocarbons are nonpolar substances, with weak intermolecular forces. Their properties are influenced by the lack of strong intermolecular attractive forces so they have the lowest boiling points among the organic compounds.
But here the number of carbon atoms are different and it is true that by increasing the number of carbon atoms, boiling point increases but here the intermolecular bonding due to functional group has a more significant impact . Also, the difference in the number of carbon atoms is not that big and thus we have B as the answer.
thank you so much!! i did not infact know about the carboxylic acids and I was pretty confused… Have a nice day!