Hello!
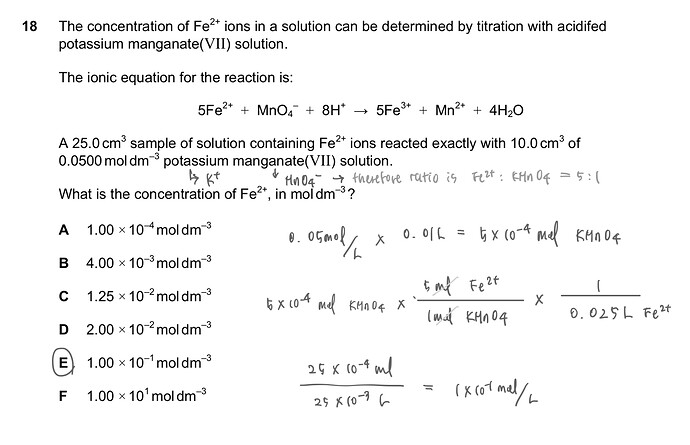
The concentration of Fe2+ ions in a solution can be determined by titration with acidifed potassium manganate (VII) solution.
The ionic equation for the reaction is:
5Fe2++ MnO4 + 8H+ 5Fe3++ Mn2+ + 4H2O
A 25.0 cm3 sample of solution containing Fe2+ ions reacted exactly with 10.0 cm3 of 0.0500 mol dm3 potassium manganate(VII) solution.
What is the concentration of Fe2+, in mol dm3 ?
Bmat 2019
A
1.00 x 10 mol dm-3
B
4.00 x 10-3 mol dm-3
C
1.25 x 10-2 mol dm-3
D 2.00 × 102 mol dm3
E
1.00 x 10-1 mol dm-3
F
1.00x101 mol dm3
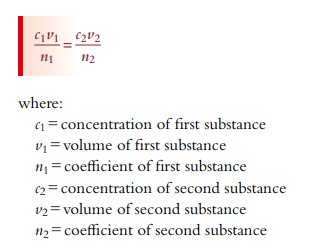
I solved it with stoichiometric ratio and got it right. But then i tried to solve it with C1V1=C2V2 formula and it didn’t work. Why was that? what are the limitations of the C1V1=C2V2 formula and when should I do use it.
thanks a lot ![]()