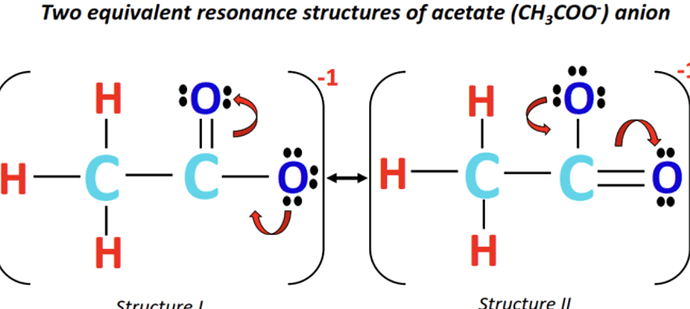
answer is B
I understand that the first one is right but how is third one correct?
everywhere i look gives me a different structure but in most i could see that one is double bond while the other one is single…
can someone explain? have any ideas?
hi
the diagram is correct, the molecule is constantly changing between the two states, either the first O carries the -1 charge, either the second one carries it
the resonance structure is an in-between of the two, we’ll consider each O atom carries a -1/2 charge, and the double bond is split between the two atoms, so their bond lengths would be the same