Hey guys!
I have a question regarding the action of catalysts.
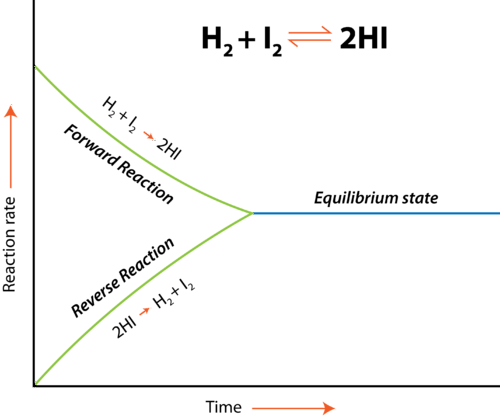
We know that catalysts increase both, the rate of forward and backward reaction, so equilibrium is reached sooner. However, when we look at the forward/backward reaction rate graph, we see the rate of forward reaction DECREASES while backward reaction increases.
Can anyone explain how does it happen that the rate of forward reaction and backward reaction increase at the same time?
Here’s the graph I’m referring to:
Thanks a lot!
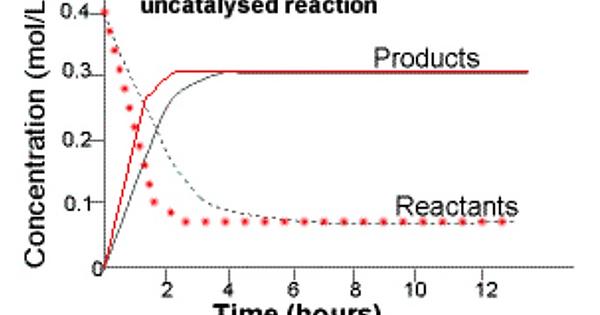
Hey! From what I’m gathering, a catalyst increases the rate of the reaction compared to the uncatalysed reaction. The forward rate still decreases over time but the slope is steeper because the activation energy has been lowered and therefore the reactants disappear faster (this is the “increase” that the catalyst causes). With that said, the natural pattern of the reactants being used up still holds. See the graph below for reference. This one looks at concentration over time but it still applies because of the relationship between concentration and rate. Hope that made sense! If anyone has any other info please include it 
4 Likes
Thank you so much Romie, really appreciate this!
So basically, the “increase in rate of forward reaction” means steeper fall in concentration of reactants over time. Makes sense!
3 Likes