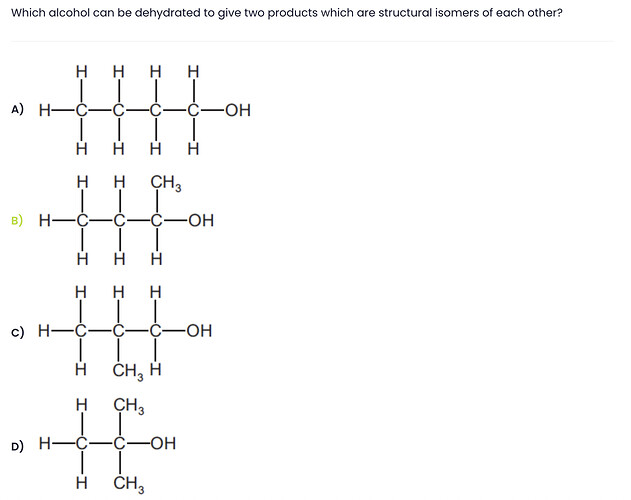
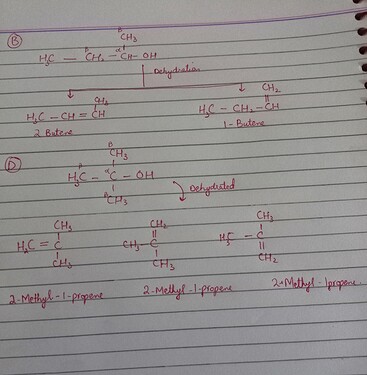
First of all you need to recall when an alcohol (-OH) is dehydrated, it forms alkenes (=).
Dehydration is an elimination process. In which OH being removed from one carbon and H from the adjacent carbon (beta-carbon). So in order to have two product there must be at least two carbon atoms adjacent to the alpha carbon.
Now you have to look for the option that gives two products.
-
We can easily eliminate the option A and C as it contains only one adjacent carbon atom.
-
Look at option D it has three carbon available to donate H-atom. But when it will be dehydrated all the products would have same structure. (See the picture attached)
-
Lastly, if we dehydrated the compound in option B (2-butanol) we will get two different products.
3 Likes