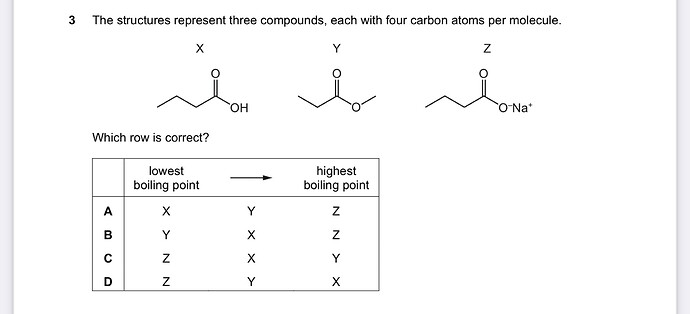
Which is the stronger bond between ion- dipole interaction and hydrogen bonds? I am a bit confused about these two
Hello! The answer key says its B
I did not pay attention to the point that the lowest boiling point was at the left
When we have a high boiling point it means that we have a higher intermolecular and interamolecular forces.
We know that ionic bond is stronger than the covalent bond and it’s a intermolecular force; NaO forms an ionic bond, and also OH forms a hydrogen bond which is a covalent bond. So the boiling point of the substance that has NaO is the highest. And the lowest boiling point refers to the substance that has no atoms attached to it to form a stronger bond.
Thank you so much for your explanation. I understand now😁 I initially thought O-Na+ bond was an ion-dipole interaction😅.
Don’t mention it!
No it’s an ionic bond cause it’s between a metal and nonmetal