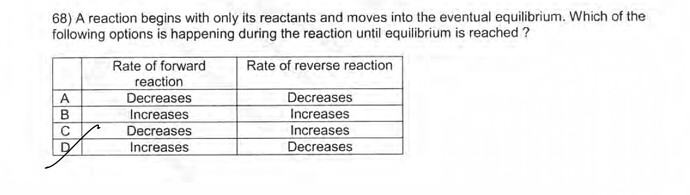
As equilibrium is approached, why is the rate of forward reaction decreases and the reverse increases ? The key guide said it’s C
Hey!
Because the forward reaction wants to reverse. So the rate of forward reaction decreases and the rate of reverse reaction increases until the equilibrium is reached.
Thanks for the answer! To my way of thinking, I thought forward reaction would be favored because there are only reactants, can it not be considered that way?
@Khinehsustella Hi!
You’re welcome!
The thing that you’re talking about is at the beginning of the reaction.
You should consider that the speed of the reaction depends on changes in the concentration of the participating substances.
In the equilibrium reaction, At t=0 , as there is the maximum amount of reactants and the amount of the products is 0, the rate of forward reaction is at it’s maximum and the rate of the reverse reaction is at it’s minimum.
But after a while, as the reaction wants to reaches equilibrium,and also since some of the reactants are consumed during the reaction and the concentration of them has been decreased the rate of the forward reaction decreases and the rate of the reverse reaction increases until it reaches the equilibrium. ( we know that the reaction wants to be reversed.)
if you Specify this message as the solution others can use it better!