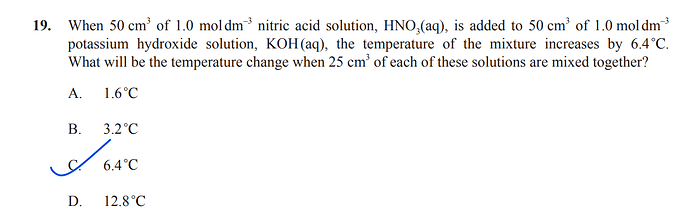
1 Like
Hi
By given values,
mol of HNO3 = 0.05 dm3 * 1 M = 0.05 mol
mol of KOH = 0.05 dm3 * 1 M = 0.05 mol
The balanced reaction for mixture,
KOH + HNO3 —> KNO3 + H2O + heat
1 mol + 1 mol —> 1 mol + 1mol + heat ( 6.4 C)
and also in latter case they didn’t change the Molarity of both solutions and halved the volumes for both.
mol of HNO3 = 0.025 dm3 * 1 M = 0.025 mol
mol of KOH = 0.025 dm3 * 1 M = 0.025 mol
KOH + HNO3 —> KNO3 + H2O + heat
1 mol + 1 mol —> 1 mol + 1mol + heat ( 6.4 C)
Therefore, this would also produce the same temperature value which is 6.4 C
1 Like