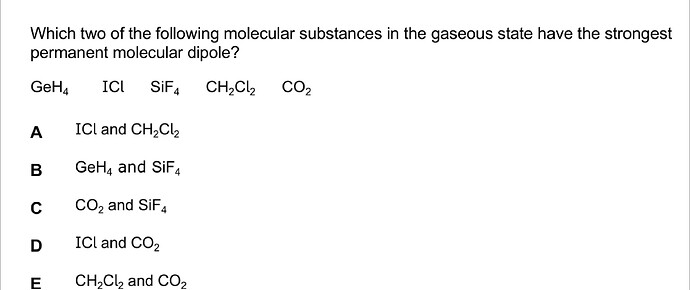Hi! Would like to get an explanation about this question and the solving process.
1 Like
Hi! we’re looking for the largest electronegativity difference here. we know that electronegativity goes about up and to the right at the periodic table, while we can see metals have very low electronegativity values, transition metals have a bit higher values and nonmetals have the highest.
we can compare H electronegativity is similar to left and down placed non metals and transition metals.
- GeH4 : H and transition metal -pretty close values
- ICl halogens have pretty close values except a big leap to F
- SiF4 : transition metal and non metal - a good electronegativity gap! especially because the non metal is F which is very high and to the right at the table.
- CH2Cl2 : non metal connected to H and 2 Cl (non metal). Cl is on the right end and top part of the table. while C is less right and less electronegative and H is the least electronegative here. for a permanent dipole we need a cis and not a trans configuration so I’m not sure this is the best option here.
- CO2 : C is less electronegative than O, the electronegative difference has very large leaps when we look at the 2nd row due to the same number of outer shells but larger atomic charge.
so the correct answer is CO2 and SiF4
I hope my explanation was clear and useful! If you have the answer please let me know if i was mistaken and i’ll try to figure it out again lol
the correct answer is A) ICl and CH2Cl2
all other molecules with cancel electronegativity differences due to geometry.
2 Likes