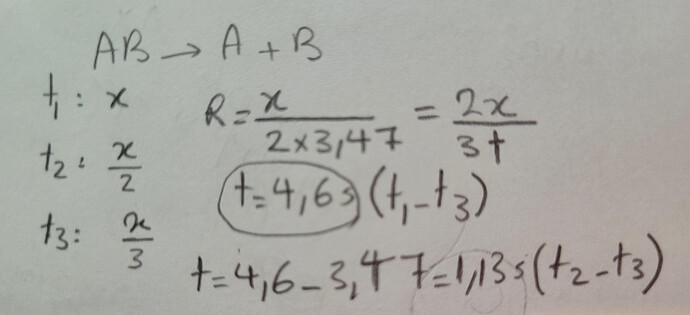- A first-order decomposition reaction is shown below.
AB(g) → A(g) + B(g)
The half-life of the reaction was found to be 3.47 s. What is the time taken for AB(g)
to reach one-third of its initial concentration?
A. 3.0 s
B. 3.5 s
C. 5.5 s
D. 7.0 s
E. 6.0s
Mind mentioning the answer?
Hey!
I think the answer must be 4.62 which is not in the question:))
c is the answer. I just need an explanation
Hey Ari, could you please also include some practice problems on “Kinetics and Catalysis” in the summer class resources?
Imgur: The magic of the Internet @AriHoresh hey!
This is my worked solution, but there is not such an option in the question…
Hi,
I think I found the explanation to this question. We know the half-life is 3.47s, so 1/3 of the original concentration is going to be reached in the “2nd half-life” (between 3.47-6.94s). We also know that 1/3 is almost half-way between 1/2 and 1/4 of the concentration (a little below). Therefore, the time to reach 1/3 of concentration will be a bit more than half-way between 3.47s and 6.94s. Thus, its a bit more than 5.205s which is the closest to 5.5s, so the answer should be C.
I hope that makes sense:)
Hey Darius!
Thanks!
But I really have no idea why my answer’s wrong!