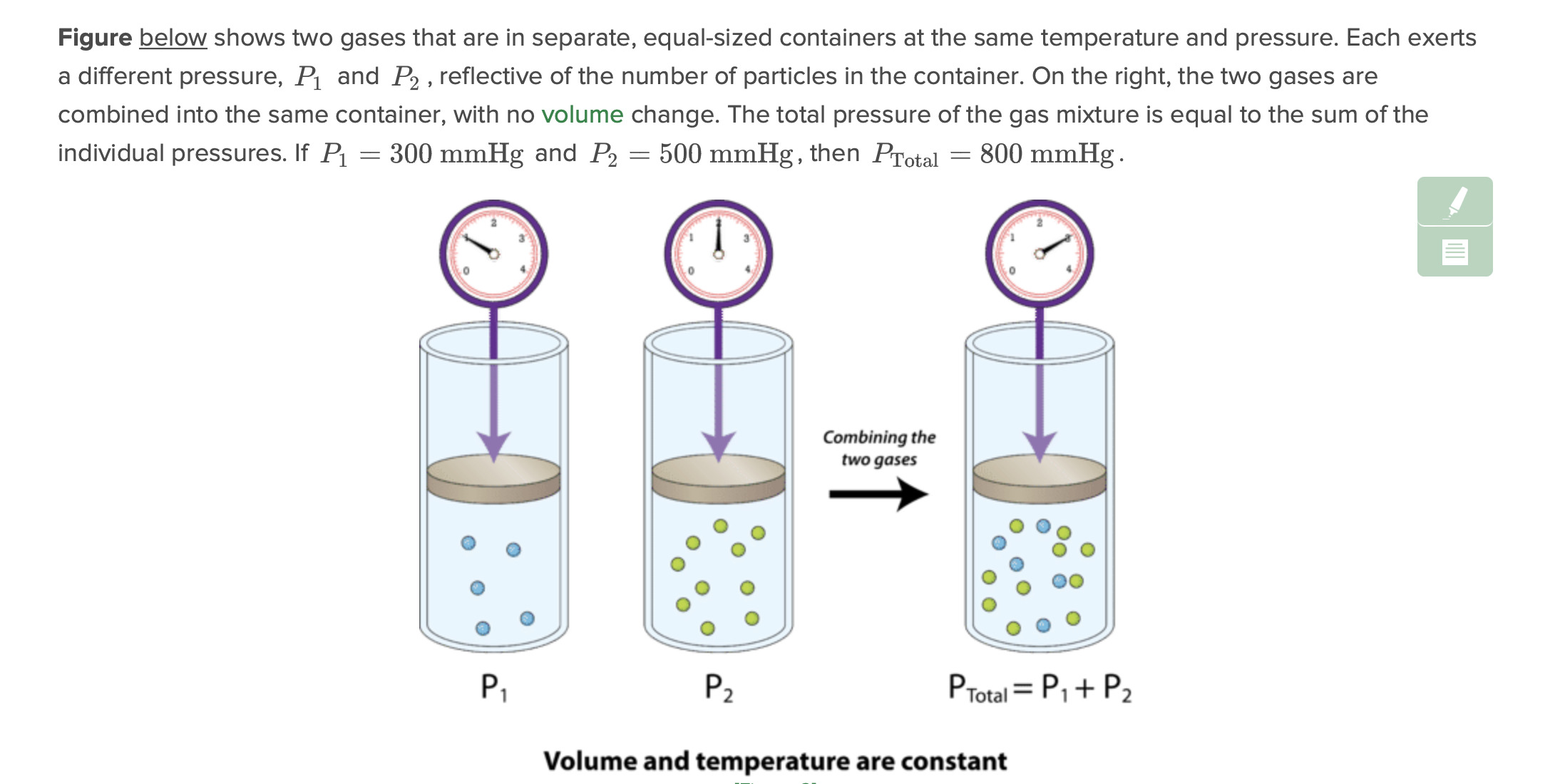
air is about 78 percent nitrogen, 21 percent oxygen, and 1 percent other gases. after all the oxygen is removed from a sample of air in a glass tube and the temperature remains constant, the pressure exerted by the remaining air
A: does not change
B: is reduced by 50 percent
C: Is reduced by 78 percent
D: is reduced by 21 percent
It’s reduced by the same percentage - 21 per cent because when there are less molecules, there are less collisions (more space between molecules) so the pressure decreases. So the answer is D.
Hope this helps!
There seems some mistake in your reasoning. @callearaya
Using PV=nRT we can understand when the volume is reduced the pressure reduces proportionally provided that the temperature remains constant!
According to the general gas equation PV=nRT, if the temperature remains constant then Pressure is inversely proportional to the Volume. (P=nRT/V)
Oh yes that’s true because this equation only applies to one ideal gas not a mixture of gasses. Thanks for the correction!
in this case what would the answer be?
The answer would be A, pressure does not change! Each gas is still in the same volume of the container, so at constant temperature it exerts the same pressure.
Omg I’m so sorry, I think what I read up must have been wrong. Probably got confused when I read that Dalton’s Law, or the Law of Partial Pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the mixture.
I think its not incorrect tho, just the question is stated kind of weirdly:D Of course the total pressure in the CONTAINER decreases when we take away one of the gases due to Daltons law. BUT, the question is asking about the pressure exerted by the remaining air, so pressure of each of the GASES inside the container is the same still:) its a little chaotic I know haha
yeah right?? i think it was because of the wrong wording but we learn new things everyday!!
That sounds helpful thank you!!
Yess exactly I knew you were right hehe just when I read it beforehand, I got it confused :))