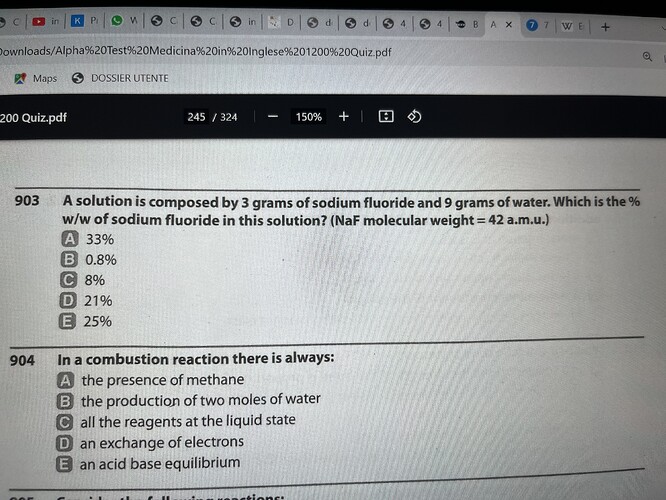
Hello how can i solve this any help ? 903
% w/w means weight per weight, so its disregarding the volume. It is the weight of solute divided by the weight of the solution (solute+solvent)
So I assume the equation would be 3/(3+9) x 100 = 25% so the answer is E
can u confirm?
1 Like
The answer is D ![]() 21%.
21%.
I’d agree with E tbh, not sure how it would get D unless theres something involving molecular weight in the calculation?
damn I have no clue then😭, anyways I don’t think its required for the imat