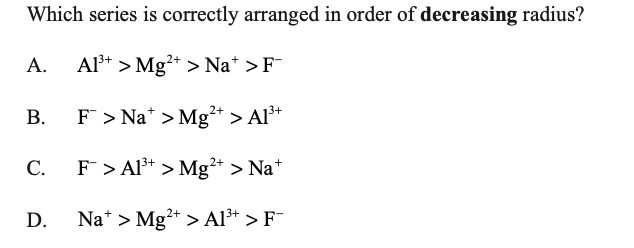
answer is B
my question is, if the electron number is the same then does that mean we check it according to the proton number?
Yes, even though the electron numbers are the same here, the nuclear charge is increasing . This increasing nuclear charge attracts the outermost electrons closer to the nucleus with increasing atomic number.
1 Like
Yes, in isoelectronic species we should keep the charges in mind. The trend is the higher positive values have smaller radius and the hishest negative values have the largest radius
In this case they asked in decreasing order so first will be the the species with negative charge and largest radium to increasing positive charges.
Hence, B is correct
1 Like