hi
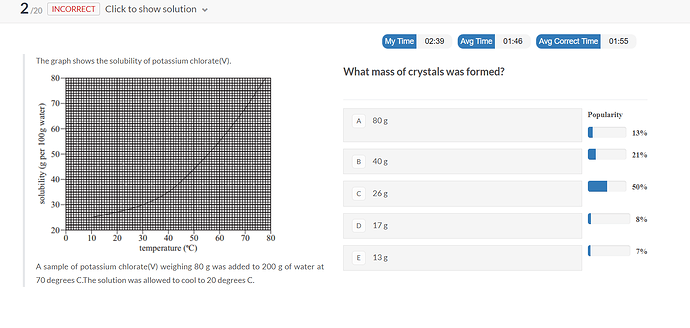
we only need to look at the final temperature to solve
at 20°C the solubility is 27g/100g of water according to the graph
so 27 * 2 = 54g of KClO3 is dissolved in the sample
80-54=26g so 26g of KClO3 is undissolved crystal
1 Like