I got like 1.677*10^21 :))
hi
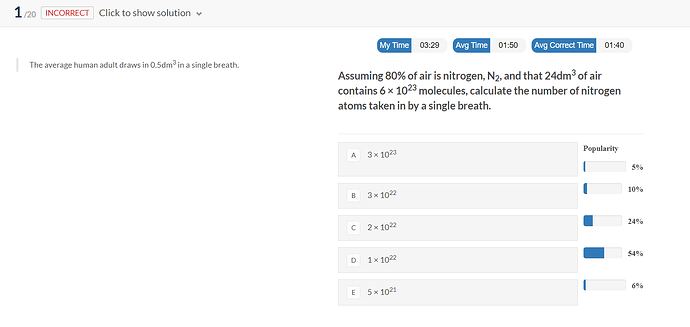
is the correct answer C)?
24L = 6 * 10²³ molecules
so 0,5L = 0,125 * 10²³ molecules
80% are N2 so there are
80 * 0,125 * 10²³/100 = 1 * 10²² molecules of N2
N2 has 2 atoms of nitrogen so 2 * 1 * 10²² = 2 * 10²²
1 Like
Yeah the correct one is C)
Thanks!!
1 Like