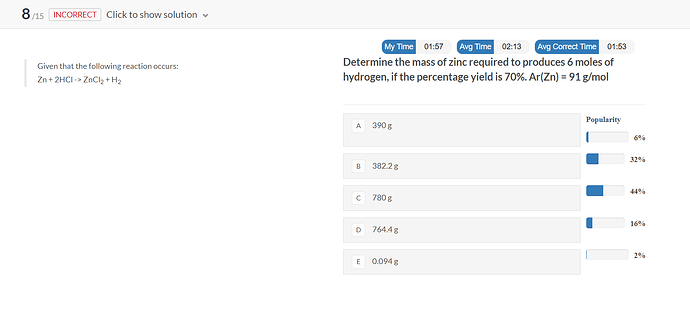
Why the answer is C?
hi
molar ratio H2 : Zn is 1 : 1 so 6 moles of H2 are produced by 6 moles of Zn
m=n * Mw = 6 * 91 = 546g
percent yield = 70% so out of our sample of Zn only 70% = 546g reacted
546 * 100 / 70 = 780g
2 Likes