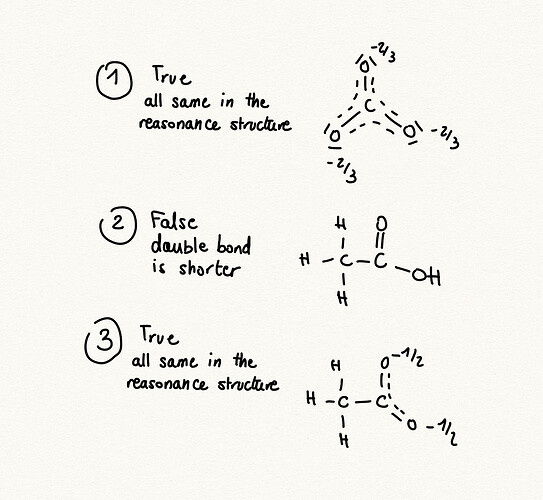
the answer is B, can someone explain?
thanks! but one question, in one shape (for the first and third one) we will have one double bond at the same time even tough they have resonance structures. then doesn’t it mean that the lengths will vary between resonance versions?
you’re right that the lengths will constantly vary, but i believe in resonance structures we consider that the molecule exists as a mix between the two structures which is why in figure 3) we can say that each O has 1/2 charge, even though in reality it is constantly changing between a charge of 0 and 1
1 Like