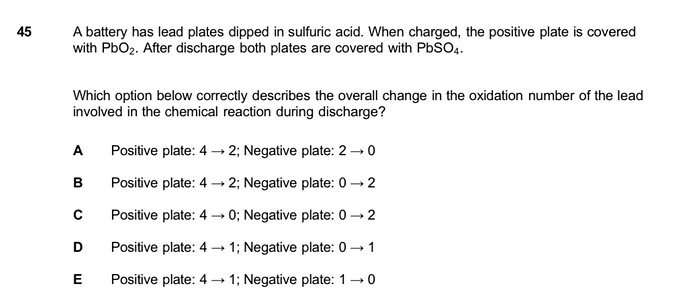
Anyone knows how to solve this?
Hi! So first we have to know that during the discharge mention, a redox reaction occurs. Reduction occurs on the positive plate while oxidation happens on the negative plate.
At the negative anode, lead is OXIDIZED from Pb to Pb 2+ (0 to 2).
At the positive cathode however, we can see it’s covered with PbO2, the oxidation number of Pb in this compound is +4. When it changes to PbSO4, the oxidation number becomes +2. Therefore, the answer is B. Hope this helps!!
But oxidation always take place at the anode (POSITIVE ) and reduction occur in the cathode (NEGATIVE). Or am i wrong? @callearaya
The Anode is the reducing electrode that releases electrons to the external circuit and oxidizes during the electrochemical reaction even though it’s positively charged, it’s known as the negative/reducing electrode. That’s why my explanation was a bit confusing haha.
You’re right though, when charged the anode is positively charged!