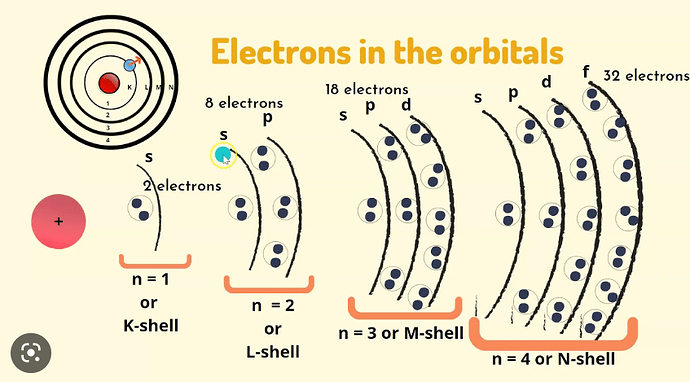
an orbital can contain a maximum of:
4 electrons
1 e
16 e
2 e
8 e
initially, I went with 8 electrons but when I searched online I said 2 electrons which confused me. the reason was that it can contain -1/2 and 1/2 spins in one orbital. can someone explain if 2 is correct?