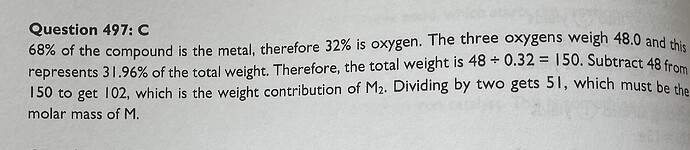Hey, can anyone help me solve this question? I have no idea what’s going on in the solution given by the book. My initial thought was to do simple calculation of 48 = 0.32 and x = 0.68 but this turned out wrong.
1 Like
- We know that the mass of 3 moles of oxygen (O) in the compound is 48 g.
- From the problem, we know that this mass of oxygen constitutes 32% of the total mass of the compound.
- Therefore, if 32% of the total mass is 48 g, we can find the total mass (which would be 100%) by dividing the known mass of oxygen by its percentage (expressed as a decimal).
So, the calculation “48 g / 0.32” gives us the total mass of the compound M2O3.
Let’s solve this problem step by step.
- The empirical formula M2O3 means that for every 2 moles of metal, there are 3 moles of oxygen.
- The atomic mass of oxygen (O) is given as 16 g/mol. Therefore, the mass of 3 moles of oxygen is 3 * 16 = 48 g.
- The problem states that the metal constitutes 68% of the total mass of the compound. This means that oxygen constitutes the remaining 32%.
- If 32% corresponds to 48 g (the mass of 3 moles of oxygen), we can calculate the total mass of the compound (100%) as follows:Total mass = 48 g / 0.32 = 150 g
- Now, knowing that the metal constitutes 68% of the total mass, we can calculate the mass of the metal:Mass of metal = 0.68 * 150 g = 102 g
- Since the empirical formula tells us that there are 2 moles of metal, the molar mass of the metal (the mass of 1 mole) is:Molar mass of metal = 102 g / 2 = 51 g/mol
1 Like