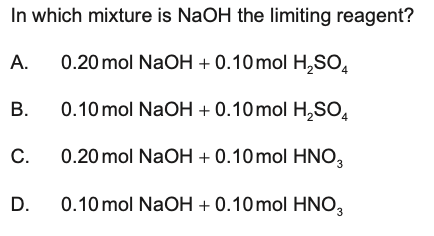
limiting reagent question answer is B
gases question is A
That first question, I think the NaOH, H2SO4 reaction you need 2NaOH for every H2SO4 so with B having the same amount for both means that NaOH is limiting the amount that could be formed.
hi
i agree, since 2NaOH + H2SO4 = NaSO4 + 2H2O
and NaOH + HNO3 = NaNO3 + H2O
in option B) we will fully consume NaOH but have an excess of 0,05 mol H2SO4, so NaOH is the limitant in this reaction
The second question is a bit tricky, a gas deviates from ideal at high P and low T
option C) is a high T so it can’t explain why the gas deviates from ideal
option B) cohesive forces would increase the volume
both A) and D) seem to be correct
i guess we can say that A) causes D), so A) explains better the root cause why gases deviate from ideal
ideal gases are assumed to be like a point with no charge and no volume, since real gases do have a fixed volume they will collide and interact. At higher pressure these collisions happen more frequently so more chances of creating IMF which means the gas no longer has ideal behavior
what confused me about the second question was option D! with more pressure it causes molecules to have higher chance of collusion and with that a better chance of not acting ideally.a tricky wording question for sure
Same tbh, am I right in thinking that under ideal gas laws Pressure is inversely proportional to Volume but under high pressure the molecules can only get squeezed together to an extent, eventually meaning that it wouldnt be inversely proportional - breaking the ideal law? And thats why it is A?
same confusion here. i believe it is the wording… lets hope they wont ask this much confusing questions on the exam lol
Maybe it’s because more collisions happen when volume is constant AND pressure increases, D might not be correct under the circumstances when the volume of container increases / temperature, # of moles decrease.