Hi idk about the first one but in the second one u forgot to add the O from H2O which instead should be a total of 14 mol of O2 in 1 mol of hydrated iron(Ii) ammonium sulphate. So 0.5 mol would have 7 mol of O2.

I don’t know much about this topic, so I did some research myself.
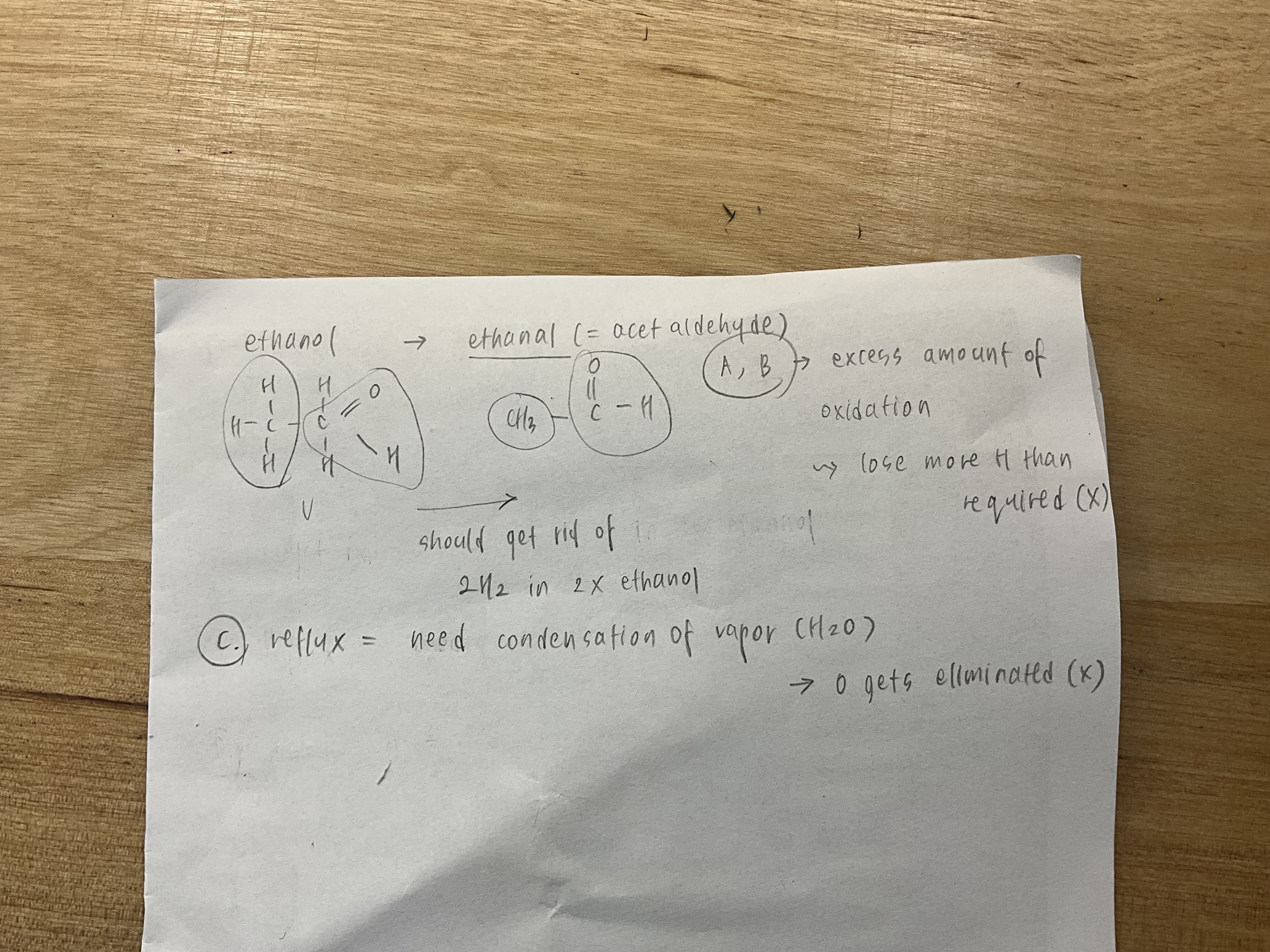
H2 gas can be elliminated using distillation.
(More on reflux: https://en.wikipedia.org/wiki/Reflux)
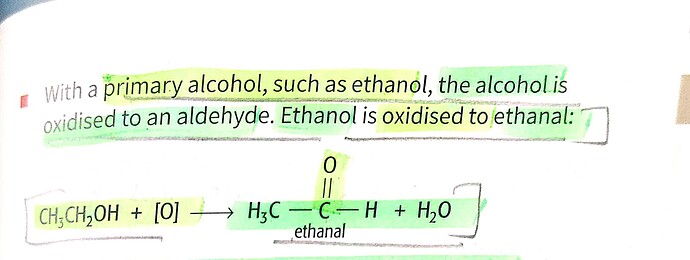
Hey, can anyone tell me why A is wrong for Q26. As far as I know, For a primary alcohol like ethanol, refluxing it with an oxidizing agent converts it to an ethanal.
This is what I learned… Am I missing out something?
Maybe it’s the keyword “excess” in A. It’s more likely to become acetic acid.
Yeah, that makes sense… Thank You
Ok so maybe Ive learnt this wrong, but i thought if you primary alcohols is oxidised by distillation it goes to an aldehyde, and then if the aldehyde oxidised by reflux it goes further to carboxylic acid? (Eliminating option A and C by that logic)
Also on: oxidation of alcohols
it says
'You get an aldehyde if you use an excess of the alcohol, and distil off the aldehyde as soon as it forms.
The excess of the alcohol means that there isn’t enough oxidising agent present to carry out the second stage. Removing the aldehyde as soon as it is formed means that it doesn’t hang around waiting to be oxidised anyway!’ - so I guess this is why???
This might be the reason. I actually thought that when the primary alcohol is ‘refluxed’ with an oxidizing agent it forms an aldehyde… That’s where i went wrong…Thank you for clearing that up!
can you explain what you mean by reflux?
Hey,here is an explanation of reflux that i found ’ Reflux is a technique used to continuously boil a reaction mixture.The vapor produced is condensed back into the vessel, preventing the loss of volatile compounds and it is carried out in a steady temperature unlike distillation, in which temperature increases and separates components of a mixture based on the boiling point.’