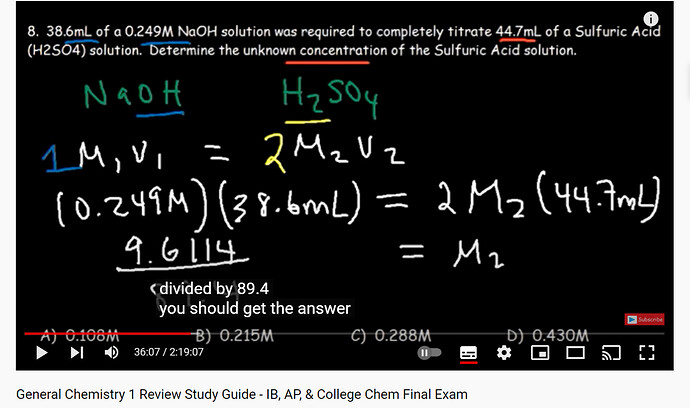
If we use formula M1V1 = M2V2
Do we have to multiply M2V2 by like 2(M2V2)
If we have 2 mole of NaOH why do we have multiply M2V2
hey! It’s not common to multiply it by 2, because the formula itself is M1V1=M2V2; but in the vid, Organic Chemistry Tutor multiplied by two because the problem includes a diprotic acid. So for 1 mol of H2SO4, we have 2 mol of H ions
oh, thanks!
and yeah right we don’t use 2 every time I was talking about this particular problem
And what if situation was different?
can you please give any example?
hey! Sorry for the delay, could you explain what you mean with this?
hey ,no problem
I meant, what if there would be 3 hydrogen
Then it will be multiplied by 3?
mmmm I think so! Anyway don’t worry too much about that, triprotic acids are quite rare in the IMAT. Diprotic acids are a common trap so while you understand how to work with diprotic acids you are gonna be more than fine ![]()
Great.![]()
Thank you so much