answer is C
doesn’t dehydration mean taking out H2O?
in this case how does ethoxyethane can occur knowing that we took out the Oxygen?
Ethoxethane would involve a secondary reaction right? Ive searched up the formula its C4H10O - so maybe another reaction where 2 ethenes and a H2O combine?
If a single ethanol is used and is dehydrated than ethene is formed
C2H5OH ----> CH2=Ch2 + H2O
If two ethanol are used then ethoxyethane is formed
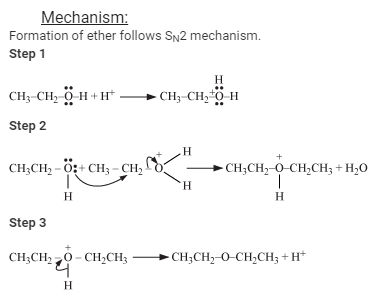
hello!
do we need to know the SN1 and SN2 mechanisms in detail?
and one thing i cannot get straight, doesn’t dehydration take out 2 hydrogen and one oxygen? or is it simply just removing hydrogen?
Hey
dehydration means we took out a water molecule not a hydrogen molecule. In both cases we took an h2o as firstly ethanol has formula C2h5OH we took a water molecule and it became C2H4 which is ethene
To make ethoxyethane we took 2 molecules of ethanol and we took oh from and H from the second thats how we got an ether
And about SN1 and SN2 I would recommend to go through it because it impacts the reactivity in haloalkanes and also the products formed
