Hi!
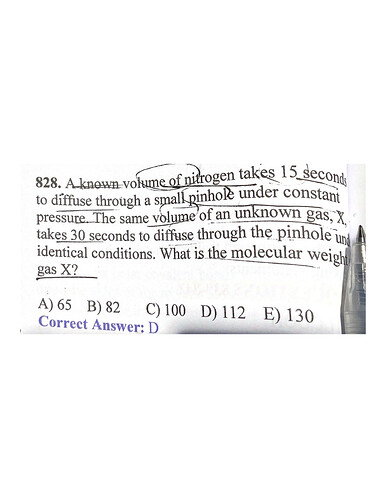
You can use the Graham’s law of diffusion to calculate the missing molar mass
t1/t2=Sq. root M1/M2
Where M2 is the molar mass of gas X.
Hope it helps!
3 Likes
Heyy, should we also know the Graham’s laws for the IMAT?
Hi Darius!
I don’t think we need to know it for the IMAT also it’s not mentioned anywhere in the specification. This is something i learnt in High school so i mentioned it anyways, no need to worry regarding it:)
2 Likes