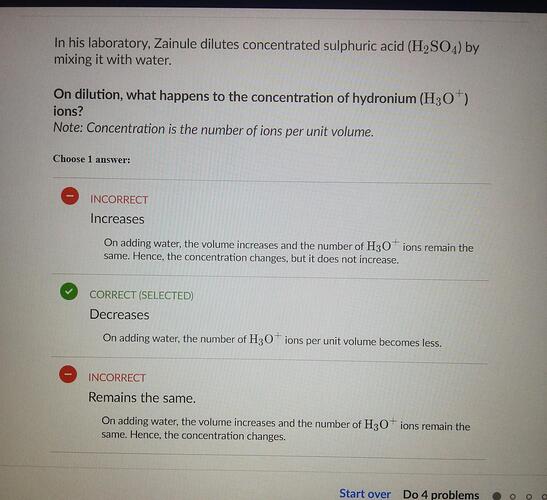
Hey, can someone please explain how these ions decreases and not increase
i didn’t get it
I thought its increasing bcoz when we add H2O and H+ it becomes hydronium ion then how come these decreases when adding water
Hi!
That’s a tricky question,
let’s imagine we start with 1 liter of 1 mol H2SO4 and we dilute it in 1 liter of water,
with 2H2O + H2SO4 ---- 2H3O⁺ + SO4²⁻
For every 1 mol H2SO4 we will get 2 mol H3O⁺.
Initial concentration = n/v = 2/1 = 2
Final concentration = 2/2 = 1 so [H3O⁺] lowers.
Hope this helps!
1 Like
Thank you so much for such a nice explanation
1 Like