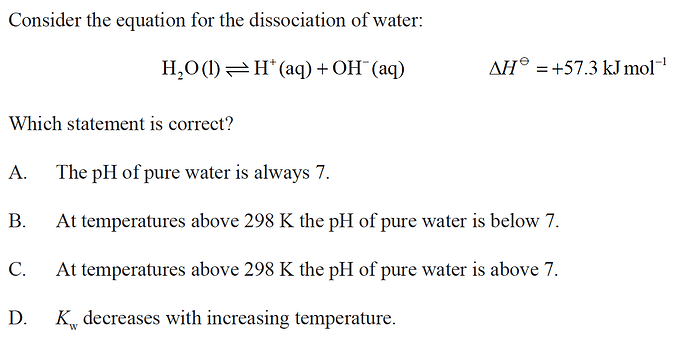
answer is B
one thing that I cant understand, so if we increase the temperature the rxn shifts to the right. this will cause an increase of both H+ and OH- concentrations to increase. but as their coefficients are the same, increase will be constant. then howcome the pH can drop if we don’t change the concentrations while keeping the other one same
yes the concentrations of both H⁺ and HO⁻ will increase which causes the pH to lower and pOH to increase proportionally, but the solution will stay neutral
1 Like