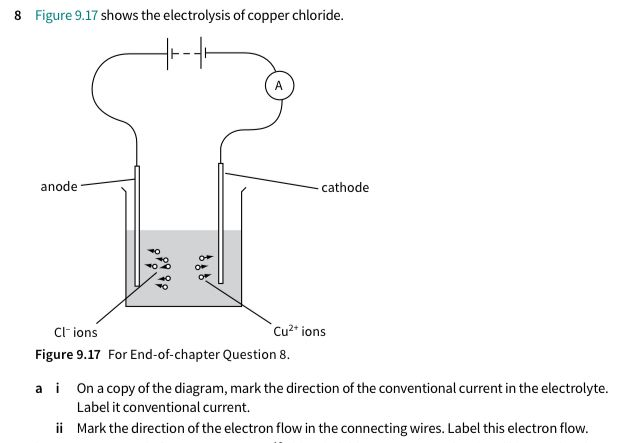

This is from Cambridge physics. It has sometimes some mistakes in the answer part. So I wonder whether a) is one of those mistakes? I got b right… but I think a) should’ve been from right to left
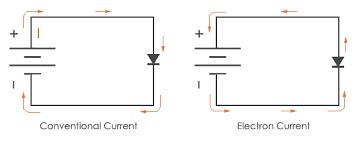
Answer credited to @Asafmen
The official answer is correct.
In electric circuits, it is assumed a positive charge without mass moves. In reality, it is the electrons that move and they have the opposite charge.