Hey Ari! I do I need to remember the Bohr and Lewis electron configuration diagram! Thanks!
Hey! Mind sharing the image over here so I can ensure we refer to the same thing? Thank you!
1 Like
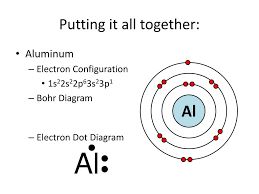
Sure ari! Thanks for replying! Let me know please!
1 Like
Yes! Everything in the illustration in important including all of the definitions you’ve mentioned!
1 Like
Hey! I can help in learning electronic configuration by a simple trick, that way you can always remember the sequence in which electrons are being filled in each subshell.
Rules:
- First write each subshell’s name and shell number in sequence
- Add one more subshell to each new shell till you got “f subshell” means no more addition of another subshell after f subshell.
- Draw an arrow in downwards direction in sequence.
You got the final electronic configuration meaning you got the sequence of how to put electrons in each subshell.
Each subshell’s electrons maximum capacity is what you have to learn only.
Final electronic configuration: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 5f 6d 6f
2 Likes
Thanks a lot Ari!![]() Wish you a great weekend!
Wish you a great weekend!
That’s so nice of you umber! It helped me a lot. Thank you🙏🏽 wish you a great weekend!
1 Like
