Hey!
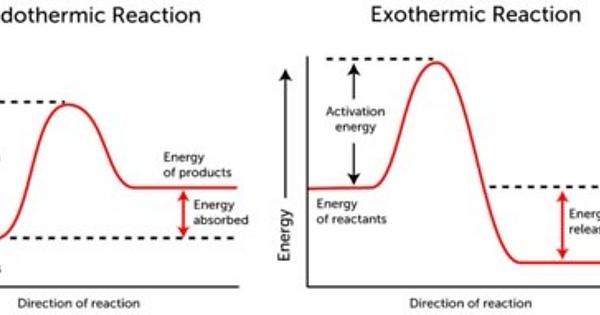
I had to look this one up. The activated complex is what forms when we add enough energy for reactant molecules to reach the activation energy of the reaction (breaking down bonds). Recalling the graphs of exothermic and endothermic reactions, the activation energy is ALWAYS above both, the reactants and products - no matter if its exothermic or endothermic. Therefore, D should be the answer.
Here are graphs, so you can imagine the situation more clearly:
4 Likes