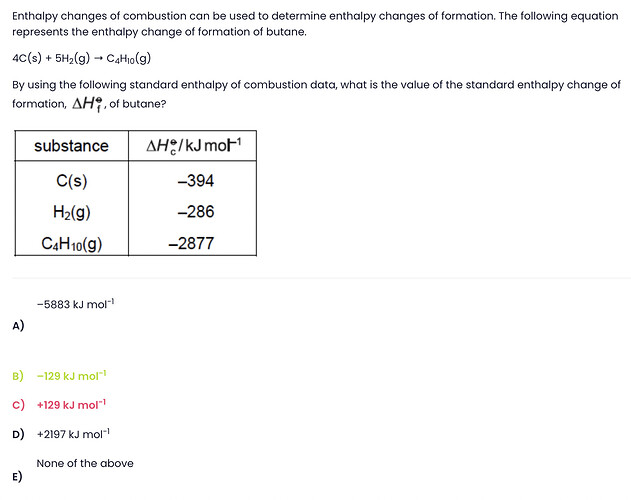
i keep finding the answer positive but is the answer negative because it should be an exothermic reaction?
Yes, a combustion reaction is always exothermic, so we see a negative change in enthalpy.
You can calculate the change in enthalpy or enthalpy of formation using this equation:
Change in enthalpy = Total Enthalpy of Reactants - Total Enthalpy of Products
3 Likes