Enthalpy change thermochemistry kinetic
@SaqibKhan Hey!
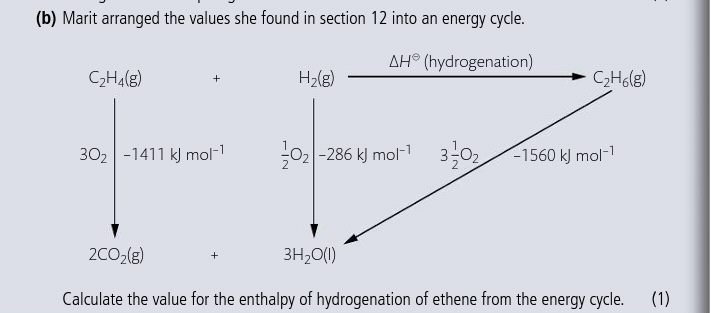
C2H4+H2–>C2H6
C2H4 + 3O2 → 2CO2 deltaH=-1411
H2 + 1/2O2 → 3H2O deltaH=-286
2CO2+3H2O → C2H6+7/2O2 deltaH=1560
C2H4+H2–>C2H6
deltaH= -1411-286+1560=-137
I believe that this qs is wrong cause the number of atoms aren’t equal on each side and this is wrong…
Hey @dorsa_vaezi @SaqibKhan , you know it’s not a part of the IMAT chemistry syllabus, right?
2 Likes
Hey !
I know. I haven’t studied this topic for the Imat. I just know it from the highschool…
I’m not sure if he’s going to take the imat exam!
1 Like