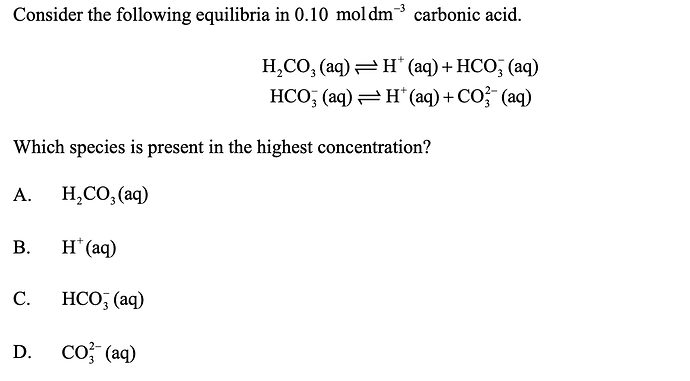
answer is A
but when i added the reactions together it comes up to
H2co3-= 2H+ + CO3-2
in this case
0.1 mol h2cho3
0.2 mol h+
0.1 mol co3-2
can someone explain how h2co3 could have more concentration
hi
this could be a question on the common ion effect
since HCO3⁻ gives H⁺, the concentration of H⁺ in the solution increases
According to Le Chatelier’s principle, increasing the concentration of the products shifts the reaction to the left
The reaction favors the left side, so the concentration of H2CO3 increases