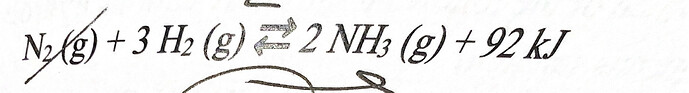Hi!
we know that at equilibrium if we increases the pressure then the side is favoured having lower no. of moles thus in this particular reaction it’s the products that have lower no. of moles compared to the reactants thus it will shift the product side.
Also, Kc depends only on the temperature and the heat of reaction whether it’s exothermic or endothermic so, pressure will have no effect on Kc value.
Answer is thus C
Hope it helps:)
4 Likes